IUPAC rules of organic nomenclature
Naming acyclic compounds
Step 1: Determining the primary functional group
a) Determine the primary functional group of the molecule.
| Ex. |

|
suffix: -ane |
This molecule contains only carbon-carbon and carbon-hydrogen bonds and therefore does not have a functional group. It is an alkane and will have the suffix –ane.
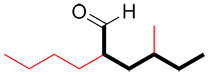
b) When there is more than one functional group present in a molecule, the group with the highest priority is usually the most oxidized one (see Appendix I for a complete list). In the example below, the primary functional group is highlighted in red. This group will be used to determine the suffix of the name. The other functional groups (if present) will be written as a prefix.
| Ex. |
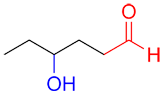
|
suffix: -al prefix : hydroxy- |
This molecule contains two functional groups, an aldehyde (in red) and an alcohol (in blue). The aldehyde has a higher priority and is therefore the primary functional group of the molecule and determines that the suffix of the name will be –al. Since the alcohol has a lower priority, the prefix hydroxyl- will be added to the name.
c) Alkenes and alkynes are never written as a prefix, they are always in the suffix. In the event there is functional group of higher priority, the alkene or alkyne should be written after the root of the name, and before the suffix of the primary functional group like so: -en- instead of –ene and -yn- instead of –yne.
| Ex. |
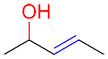
|
The alcohol (in red) is the primary functional group of this molecule; the suffix is therefore –ol. Since the alkene does not have the highest priority but cannot be a prefix, the suffix –enol is given to the molecule.
Step 2: Determining the primary carbon chain
a) The primary carbon chain is the longest carbon chain in the molecule, which contains the primary functional group.
| Ex. |
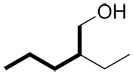
|
instead of |
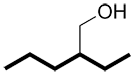
|
or |

|
b) If there is more than one option, choose the chain with the maximum number of multiple bonds (double, triple).
| Ex. |
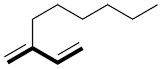
|
instead of |
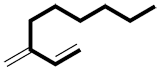
|
or |
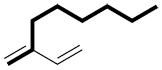
|
c) If there are two chains of the same length, choose the one with the maximum number of substituents.
| Ex. |
|
instead of |

|
| 2 substituents | 1 substituent |
Step 3: Naming the primary carbon chain
Name the primary carbon chain as well as the primary functional group. Give the same name to the chain as if it was an alkane (see Appendix II) but replacing the ending –ane with the suffix attributed to the primary functional group.
| Ex. |
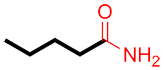
|
|
Step 4: Numbering the primary carbon chain
a) Number all of the carbons in the primary chain consecutively so that the primary functional group has the lowest possible number.
| Ex. |
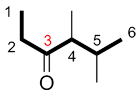
|
instead of |
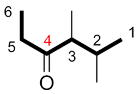
|
b) If the number for the primary functional group is the same when numbering in both directions, number the chain so the other substituents also have the lowest possible number.
| Ex. |
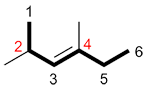
|
instead of |
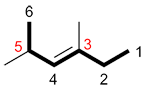
|
| Ex. |
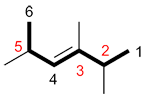
|
instead of |
|
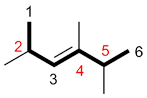
c) If the numbers for the substituents are the same in both directions, number the chain so the first substituent in alphabetical order has the smaller number.
| Ex. |
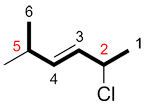
|
instead of |
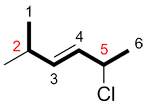
|
| "2-chloro..." | "2-methyl..." |
Step 5: Indicating the position of the primary functional group
Indicate the position of the primary functional group in between the root of the name and the suffix (see "Note well" for exceptions).
| Ex. |
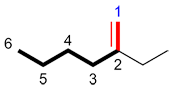
|
|
Step 6: Naming the substituents
Name the alkyl groups, the alkyl halides and any other substituents, and determine their positions on the primary carbon chain using the numbering established in step 4. Alkyls are alkane derivatives (an alkane with one less hydrogen atom). To name them, use the root of the alkane (determined by the number of carbons) and add the ending –yl. A 1 carbon branch on the primary chain would therefore be named "methyl".
| Ex. |
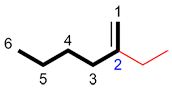
|
|
For the substituent we therefore obtain the name "2-ethyl"
This logic is also applied to for alkoxy groups (ethers). Ethers have the structural formula –OR, the R being an alkyl. It is named as mentioned above although instead of using the ending –yl, the ending –oxy is applied.
Step 7: Assigning configurations
Assign configurations to stereocentre (see Appendix V) and to double bonds (see Appendix IV):
a) Di-substituted alkenes can be named with the cis/trans or the E/Z nomenclature; however the E/Z nomenclature is always preferred.
| Ex. |

|
and |
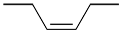
|
| (E) ou trans | (Z) ou cis |
b) Tri- and tetrasubstituted alkenes can only be named with the E/Z nomenclature system.
| Ex. |
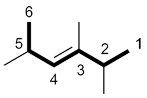
|
(E) |
c) The stereogenic carbons are assigned a configuration of either (R) or (S).
| Ex. |
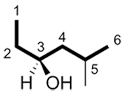
|
et |
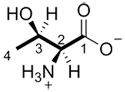
|
| (S) | (2S,3R) |
d) When both the R/S and the E/Z nomenclature are present, they are written in parenthesis, in italics when typed, with the numbers in ascending order. The parentheses are followed by a hyphen and the rest of the molecule name.
| Ex. |
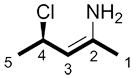
|
(2Z,4R)- |
Step 8: Assembling the entire name
ÉWrite out the entire name of the molecule, including the numbers for the substituents, and listed in alphabetical order. Do not forget to include configurations when applicable.
a) Include the multiplicative prefixes (see Appendix III) for identical substituents. However, these prefixes have no effect on the alphabetical order of the substituents.
b) Separate the position indicating numbers with colons
c) Use hyphens to join the name (root, prefixes and suffix) to the numbers
Assembled IUPAC name:
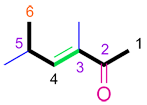
|
|
| (E)-3,5-dimethylhex-3-en-2-one |
Naming cyclic compounds
Step 1: Determining the primary functional group
a) Determine the primary functional group of the molecule.
| Ex. |
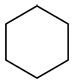
|
suffix: -ane |
This molecule contains only carbon-carbon and carbon-hydrogen bonds and therefore does not have a functional group. It is an alkane and will have the suffix –ane.
b) When there is more than one functional group present in a molecule, the primary group is the one with the highest priority, i.e. the most oxidized (see Appendix I). In the example below, the primary functional group is highlighted in red. This group will be used to determine the suffix of the name. The other functional groups (if present) will be written as a prefix.
| Ex. |
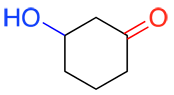
|
suffix: -one prefix: hydroxy- |
This molecule contains two functional groups, a ketone (in red) and an alcohol (in blue). The ketone has a higher priority and is therefore the primary functional group of the molecule and determines that the suffix of the name will be –one. Since the alcohol has a lower priority, the prefix hydroxyl- will be added to the name.
c) Alkenes and alkynes are never written as a prefix, they are always in the suffix. In the event there is functional group of higher priority, the alkene or alkyne should be be written after the root of the name, and before the suffix of the primary functional group like so: -en- instead of –ene and -yn- instead of –yne.
| Ex. |
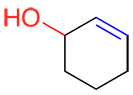
|
The alcohol (in red) is the primary functional group of this molecule; the suffix is therefore –ol. Since the alkene (in blue) does not have the highest priority but cannot be a prefix, the suffix –enol is given to the molecule.
Step 2: Determining the primary carbon chain
When the primary functional group is on a cyclic carbon chain, identify the ring as the primary carbon chain.
| Ex. |
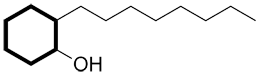
|
instead of |
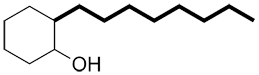
|
| Ex. |
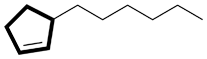
|
instead of |
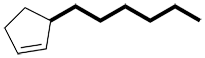
|
Step 3: Naming the primary carbon chain
NName the primary carbon chain as well as the primary functional group. Give the same name to the chain as if it was an alkane (see Appendix III) but replacing the ending –ane with the suffix attributed to the primary functional group. Add the prefix cyclo- to the name.
| Ex. |
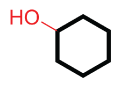
|
|
| cyclohexanol |
Step 4: Numbering the primary carbon chain
a) When the ring is mono-substituted, le carbon bound to the primary functional group is always carbon 1 (C1). In the case of a mono substituted ring, it is not necessary to indicate the position in the name.
| Ex. |

|
cyclopentanol |
b) When a cyclic chain is poly-substituted, the C1 and direction of the numbering are determined by ensuring the lowest possible numbers are given to the following conditions, in consecutive order:
i. The carbon bound to the primary functional group is C1 and the numbering continues in the direction ensuring the lowest possible numbers for the other substituents.
| Ex. |
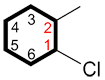
|
instead of |
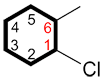
|
and |
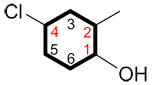
|
instead of |
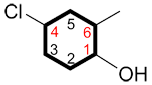
|
ii. When naming a cycloalkene, in which the alkene is the primary functional group, it is attributed C1. It is not necessary to include this position in the name.
| Ex. |
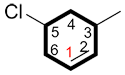
|
5-chloro-3-methylcyclohexene |
iii. When a functional group of higher priority than the alkene is present, the carbon bound to this functional group is C1 and the numbering continues in the direction ensuring the lowest possible number to the alkene.
| Ex. |
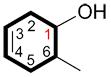
|
6-methylcyclohex-3-enol |
iv. If the numbering is the same in both directions, attribute the lowest possible number to the first substituent written in the name (alphabetical order).
| Ex. |
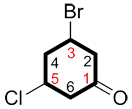
|
instead of |
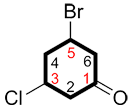
|
| 3-bromo-5-chlorocyclohexanone | 5-bromo-3-chlorocyclohexanone |
Step 5: Indicating the position of the primary functional group
Since the primary functional group is always on C1, it is not necessary to indicate the position in the name. However, the position of any other functional group(s), whether in the suffix (alkenes) or in the prefix, must be indicated.
Step 6: Naming the substituents
Name the alkyl groups, the alkyl halides and any other substituents, and determine their positions on the primary carbon chain using the numbering established in step 4. Alkyls are alkane derivatives (an alkane with one less hydrogen atom). To name them, use the root of the alkane (determined by the number of carbons) and add the ending –yl. A 1 carbon branch on the primary chain would therefore be named "methyl". For more details, please refer to step 6 in naming acyclic compounds.
| Ex. |
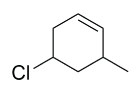
|
5-chloro-3-methylcyclohexene |
Step 7: Assigning configurations
Assign configurations to stereocentre (see Appendix VI):
Substituted cycloalkanes can be named using the cis/trans or the R/S nomenclature, however the R/S nomenclature is more precise and should be used when possible.
| Ex. |
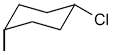
|
and |
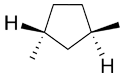
|
| cis (R/S is not applicable here; i.e. no stereocentre) |
trans or (R,R) (R,R) is more precise since (S,S) is also trans. |
Step 8: Assembling the entire name
Write out the entire name of the molecule, including the numbers for the substituents, and listed in alphabetical order. Do not forget to include configurations when applicable.
a) Include the multiplicative prefixes (see Appendix IV) for identical substituents. However, these prefixes have no effect on the alphabetical order of the substituents.
b) Separate the position indicating numbers with colons
c) Use hyphens to join the name (root, prefixes and suffix) to the numbers
Assembled IUPAC name:
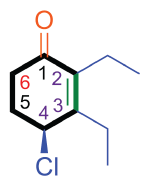
|
|
| (S)-4-chloro-2,3-diethylcyclohex-2-enone |
Naming benzene derivatives
In general
Generally, variations of benzene (cyclohexa-1,3,5-triene) are named in the same manner as other cyclic compounds and by replacing the root of the name from "cycloalkane" and "cycloalkene" to "benzene".
| Ex. |
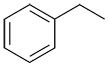
|
ethylbenzene |
2 substituents
When the benzene derivative has two substituents, the ortho (o), meta (m) and para (p) nomenclature can be used to indicate the position of the substituents.
| Ex. |
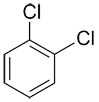
|
o-dichlorobenzene or 1,2-dichlorobenzene |
| Ex. |
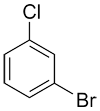
|
m-bromochlorobenzene or 1-bromo-3-chlorobenzene |
| Ex. |
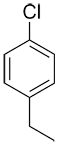
|
p-chloroethylbenzene or 1-choro-4-ethylbenzene |
>2 substituents
When there are more than two substituents on the benzene derivative, it is named by indicating the position of all the substituents according to the numbering established. Do not forget that when naming with multiple substituents, they must be written in alphabetical order.
| Ex. |
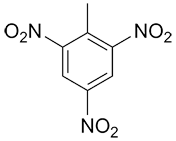
|
1-methyl-2,4,6-trinitrobenzene or 2,4,6-trinitrotoluene |
Common names
For some benzene derivatives, the common name is accepted and typically used instead of the IUPAC name.
| Ex. |
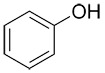
|
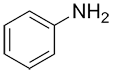
|
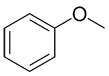
|
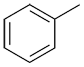
|
| Common: | phenol | aniline | anisole | toluene |
| IUPAC: | benzenol | benzenamine | methoxybenzene | methylbenzene |
| Other accepted names: | hydroxybenzene | aminobenzene | phenoxymethane | phenylmethane |
Note well
Exceptions to step 5
It is not necessary to indicate the position of the primary functional group when it can only be bound to C1, in other words it is a terminal functional group. This is the case for carboxylic acids, esters, amides, and aldehydes. In addition, it is not necessary to indicate the position of the primary functional group when only one position is possible.
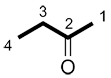
| Ex. |
|
and |
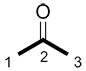
|
| butanone | propanone |
Complex substituents
A complex substituent is named following the same nomenclature rules as if it was an independent molecule. The numbering of the substituent begins with the carbon bound to the primary carbon chain as C1. The suffix –yl is given to the name for the substituent and this name is written in parenthesis in the complete name of the molecule.
| Ex. |
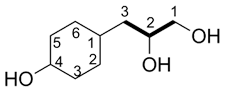
|
| 3-(4-hydroxycyclohexyl)-propane-1,2-diol |
Appendices
Appendix I: Functional group priority
| Functional group | Formula | Suffix (when primary) | Prefix (when secondary) | |
|---|---|---|---|---|
|
Carboxylic acid |
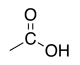
|
-oic acid | carboxy- |
| Ester |
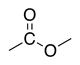
|
(R) -oate | (R)-oxycarbonyl | |
| Acid halide |
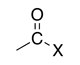
|
-oyl halide | halocarbonyl- | |
| Amide |
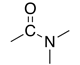
|
-amide | carbamoyl- | |
| Nitrile |
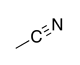
|
-nitrile | cyano- | |
| Aldehyde |
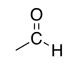
|
-al | oxo- | |
| Ketone |
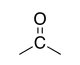
|
-one | oxo- | |
| Alcohol |
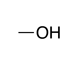
|
-ol | hydroxy- | |
| Thiol |
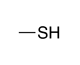
|
-thiol | sulfanyl- | |
| Amine |
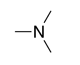
|
-amine | amino- | |
| Imine |
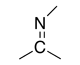
|
-imine | (R)-imino | |
| Ether |
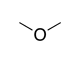
|
— | (R)-oxy- | |
| Sulfide |
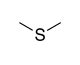
|
— | (R)-sulfanyl | |
| Alkene |
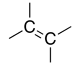
|
-ene | — | |
| Alkyne |
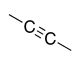
|
-yne | — | |
| Haloalkane |
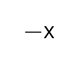
|
— | *halo- | |
| Nitro |
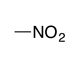
|
— | nitro- |
* Br: bromo, Cl: chloro, F: fluoro, I: iodo
Appendix II: Straight-chain alkanes
| Number of carbons | Name | Number of carbons | Name |
|---|---|---|---|
| 1 | Methane | 11 | Undecane |
| 2 | Ethane | 12 | Dodecane |
| 3 | Propane | 13 | Tridecane |
| 4 | Butane | 14 | Tetradecane |
| 5 | Pentane | 15 | Pentadecane |
| 6 | Hexane | 16 | Hexadecane |
| 7 | Heptane | 17 | Heptadecane |
| 8 | Octane | 18 | Octadecane |
| 9 | Nonane | 19 | Nonadecane |
| 10 | Decane | 20 | Eicosane |
Appendix III: Multiplicative prefixes
| Number of identical substituents | Prefix |
|---|---|
| 2 | di |
| 3 | tri |
| 4 | tetra |
Appendix IV: E/Z nomenclature
In addition to the cis/trans nomenclature, the E/Z nomenclature can also be used to distinguish between di-substituted alkenes. However, the E/Z nomenclature is more precise and preferable since it can be used regardless of whether the alkene is di-, tri- or tetra-substituted.
The "Z" is from the German word "zusammen" meaning "together" whereas the "E" is from the German word "entgegen" meaning "opposite".
This nomenclature is determined based on the position of the substituents on the carbons of the alkene (these carbons are highlighted in red in the example below).
| Ex. |
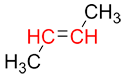
|
Step 1:
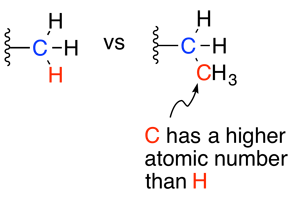
To begin, identify the primary substituent on each of the carbons of the alkene (the carbons participating in the double bond). The primary substituent is the one with the highest atomic number.
| Ex. |
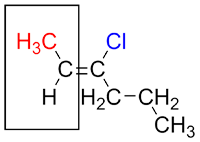
|
For the carbon on the left, the substituents are a methyl group and a hydrogen atom. The primary substituent is the methyl group because its first atom (a carbon) has a higher atomic number than the hydrogen atom.
| Ex. |

|
Now looking at the carbon on the right, its substituents are a propyl group and a chlorine atom (a halogen). The primary substituent is the chlorine atom since it has a higher atomic number than the carbon, the first atom of the propyl group.
Step 2:
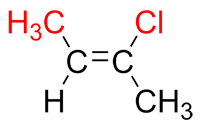
Compare the position of both primary substituent groups.
If they are on the same side of the double bond, they are "together" and are therefore in the Z conformation.
| Ex. |
|
If they are on different sides of the double bond, they are "opposite" and are therefore in the E conformation.
| Ex. |
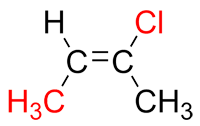
|
Appendix V: R/S nomenclature
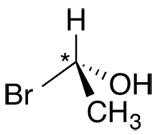
The R/S nomenclature is used to distinguish between enantiomers; i.e. configurational isomers whose atoms have a different spatial arrangement. These isomers have an atom, typically carbon, which is asymmetrical (without a plane of symmetry; bound to 4 different substituents).
The R and S are derived from the Latin words "rectus" and "sinistra" meaning "right" and "left", respectively.
| Ex. |
|
A stereogenic carbon (*) indicates that it is asymmetrical.
Step 1:
a) Assign a priority (1 to 4, 1 being the highest priority and 4 being the lowest) to all four substituents of the asymmetrical carbon. The priority is determined according to the atomic number; the highest priority being the atom with the highest atomic number.
| Ex. |
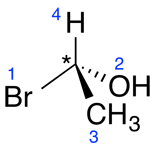
|
|
b) When the priority cannot be determined based on the atomic number of the atoms directly bonded to the asymmetrical carbon (part a) of step 1), continue this process with the second series of atoms in the substituents until a difference can be found.
| Ex. |
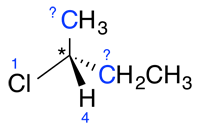
|
|
|
|
Step 2:
Rotate the molecule so that the substituent with the lowest priority (4) is at the back of the molecule (going into the page).
| Ex. |
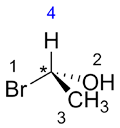
|
to |
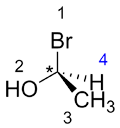
|
| Ex. |
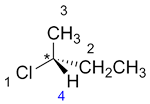
|
to |
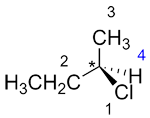
|
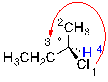
Step 3:
Trace a path from 1 - 2 - 3. If the path is going in a clockwise direction, the molecule has an R configuration.
| Ex. |
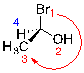
|
If the path is going in a counter clockwise direction, the molecule has an S configuration.
| Ex. |
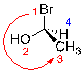
|
|
Appendix VI: Other common names
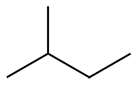
When naming alkanes, it is understood that we are referencing the straight-chain conformation of the molecule. By using "n" (in italics when typed) followed by a hyphen and the rest of the name, it is clear the molecule is linear and not a constitutional (or structural) isomer.
Ex. 2-methylbutane and 2,2-dimethylpropane are constitutional isomers of pentane since they are each comprised of 5 carbon atoms. They are also commonly known as isopentane and neopentane, respectively.

|
|

|
| n-pentane | 2-methylbutane | 2,2-dimethylpropane |
| (pentane) | (isopentane) | (neopentane) |
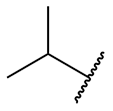
There are also some common names used to identify the different branches (alkyls) that can be found on primary carbon chains.
| isopropyl = 1-methylethyl |
|
| isobutyl = 2-methylpropyl |
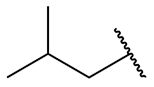
|
| sec-butyl = 1-methylpropyl |
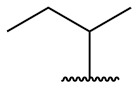
|
| tert-butyl = 1,1-dimethylethyl |

|
| neopentyl = 2,2-dimethylpropyl |
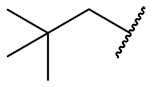
|
In this case, the alphabetical order of the substituent is affected the prefixes, except by those followed by a hyphen. The abbreviations s- (for sec) and t- (for tert) are also accepted.



